Description
Hydrated potassium aluminum sulfate (KAl(SO4)2·12 H2O). It occurs naturally when an argillaceous rock, commonly a shale, containing marcasite or pyrite which, as it decomposes, forms sulfuric acid that attacks the shale and produces alum. Often used medically as a styptic.
Related Subjects
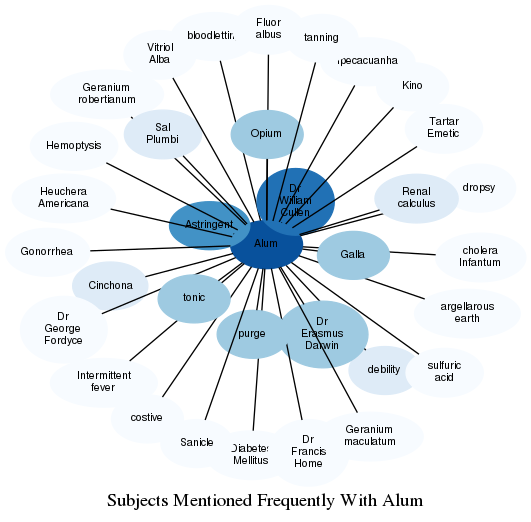
The graph displays the other subjects mentioned on the same pages as the subject "Alum". If the same subject occurs on a page with "Alum" more than once, it appears closer to "Alum" on the graph, and is colored in a darker shade. The closer a subject is to the center, the more "related" the subjects are.