Pages
46
This page is not transcribed, please help transcribe this page
47
This page is not transcribed, please help transcribe this page
48
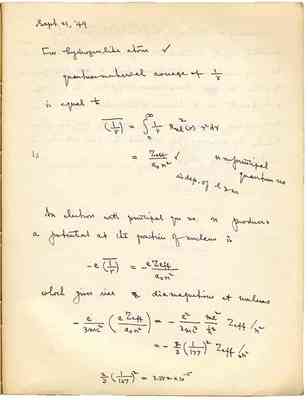
According to Slater (Phys. Rev. 36 (underlined), 57, 1930) the screening effect is taken into account in such a way that the SoP (?) electrons in (?) the same shell n are grouped together to have same effective Z. Zeff is different for d electrons with the same shell. The (?) diamagnifism at nucleus can be obtained from the above formula by summing the effective Z from all electrons combined in the atoms.
49
20Ca 1s2 2s2 2p6 3s2 3p6 4s2 1s2 Zeff=20-0.35=19.65 20-1.70-7x0.35= 2s22p6 20-4.15=15.85 3s23p9 20-11.25=8.75 4s2 20-17.15=2.85 Sum of Zeff of diff shells =19.65+15.85+4/9X8.75+2.85/16 =39.57 Diamagntic conectim factor=2/3(1/137)2[?]Zeff =3.552x10-5[?]Zeff =1040x10-3 0.259 Haitrss value 1041x10-3 Thomas Ferris value 1073x10-3 Total Zff Z for [?K]=37.08
50
26Fe 1s2 2s2 3s2 3p6 3d6 4s2 1s2 2eff=26-0.35=25.65 2s22p6 26-4.15=21.85 3s23p6 26-11.25=14.75 3d6 26-18x1.00-5x0.35=6.35 4s2 26-10x1.00-14x0.85-0.35=26-22.25=3.75 Sum of Zeff=25.65+21.85+4/9x14.75+3/9x6.35+1/16x3.85 =56.19 amegnetic curectim=3.552x10-5x56.19=2.00x10-3 haitree value=2.02x10-3 thomas ferris value=2.46x10-3 0.259