Pages That Need Review
Research Notes I, 1949
123
6/7/50
The width of the two resonance lines of Co59 in Na3[Co(NO2)6] was measured by recording the derivative of the v-mode at very small Hm ~ 0.1 G. Double Hm will give double amplitude.
Measured width = 0.72 G
Amp Ratio of the two line = 16 (low freq / high freq)
[Break]
Width of SbCl6- = 0.96 G [Width of] SbCl5 = 8.68 G
at H0 = 6673 G
121
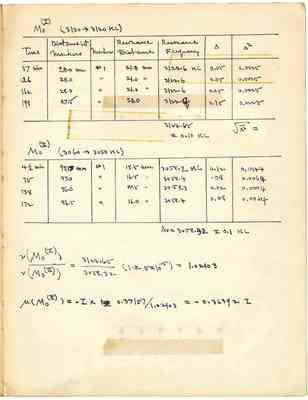
Mo(I) (3130 --> 3120 KC)
| Time | Distance bet. Markers | Marker | Resonance Distance | Resonance Frequency | Δ | Δ² |
|---|---|---|---|---|---|---|
| 37 min | 28.0 mm | #1 | 21.0 mm | 3122.6 KC | 0.05 | 0.0025 |
| 126 | 28.0 | " | 21.0 " | 2122.6 | 0.05 | 0.0025 |
| 162 | 28.0 | " | 21.0 " | 3122.6 | 0.05 | 0.0025 |
| 198 | 27.5 | " | 20.0 | 3122.[4?] | 0.15 | 0.0225 |
| 3122.6 ± 0.10 KC |
| 4 1/2 min | 95.0 mm | #1 | 18.5 mm | 3058.2 KC | 0.12 | 0.0144 |
|---|---|---|---|---|---|---|
| 75 | 97.0 | " | 16.5 " | 3058.4 | .08 | 0.0064 |
| 138 | 96.0 | " | 17.5 " | 3058.3 | 0.02 | 0.0004 |
| 172 | 96.5 | " | 16.0 " | 3058.4 | 0.08 | 0.0064 |
| Av = 3058.32 ± 0.1 KC |
115
Atomic wt of nitrogen in 31.4% enriched N15 = 14.00751 × 0.686 + 15.00489 × 0.314 = 14.321
Gauss of HNO3 soln = 12.9 containing 10.57% nitric acid by wt.
Gauss of HNO3 = 0.1057 × 12.9 = 1.3635
Moles of HNO3 = 1.3635/63.329 = 0.02153
Molecular wt of NaNO3 = 85.32
Wt. of NaNo3 = 85.32 × 0.02153 = 18.34 1.834 grams
Soln of NaNO3 = 3.5 c.c.
Molar of soln =
108
HSbCl6 was prepared by treating chemically pure SbCl₅ with 10% (~3 N) hydrochloric acid, both of the reagents were precooled to the temp of ice water. [left margin]a fuming liquid[/margin]
HSbCl6 is unstable at room temperatures Signal amplitude was reduced to about 20% after standing for 24 hours.
Suggestion: mean life time of the compound can be measured with the spectrometer.
100
Feb. 13, '50
N14 occurs at 3.34 MC at H0 = 10600 G in K3Co(CN)6. Search for N14 in Na3Co(NO2)6 in freq range 3.445 MC - 3.26 MC is negative
As to the structure of NaSbF6 refer to Nils Schrewelius, Röntgenuntersuchung der Verbindungen NaSb(OH)6, NaSbF6, NaSbO3 und gleichartiger Stoffe. Zeitschrift für anorganische und allgemeine Chemie Bd 238, 248 (1938)
NaSbF6 is cubic a = 8.18 Å The structure is probably the space group 4 Na in 4(b) , 4 Sb in 4(a), 24 F in 24(d) ; x = -0.05, y = -0.05 , z = 0.225
within the SbF6- octahedron 2.67, 2.78 and from octahedron [?] octahedron 2.72 Å
92
The function at its poles inside the unit circle around the origin.
The zeros of are the zeros of the two equations
If the zeros of (1) are z1 & z2, the zeros of (2) are the complex conjugates . If z1 lies inside the unit circle, so does . Therefore z1 & are the only two [principle?] poles inside the circle. Thus
The residue of f(z) at
The residue of f(z) at