Pages That Need Review
Research Notes I, 1949
52
55cs 1s2 2s2 2p6 3s2 3p6 4d10 4s2 4p6 4d10 5s2 5p6 6s 1s2 Zeff=55-0.35=54.65 2s22p6 55-4.15=50.85 3s23p6 55-11.25=50.85 3d10 55-21.15=33.85 4s2sp6 55-24.35=30.65 4d10 55-36x1.00-9x0.35=55-39.15=15.85 5s25p6 55-28x1.00-18x0.85-7x0.35=55-45.75=9.25 6s 55-46x1.00-8x0.85=55-52.8=3.2 Sum of Zeff=54.65+50.85+4/9x43.75+5/9x33.85+1/4x30.65 +5/16x15.85+4/25x9.25+1/72x3.2 =157.87 Dia curtium=3.552x10-5x157.4=5.60x10-3
55
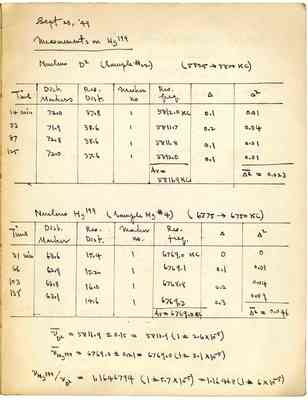
Sept 23 49 Measurements on HS199 Nuclevs D2 (Sample#22) (5825->5800Kc) Time Dist Markers Res Dist Marker no Res freg [?] [?] 14 min 7210 3708 1 5812.0kc 53min 71.9 38.6 1 5811.7 0.2 0.04 87 72.8 38.6 1 5811.8 0.1 0.01 125 72.0 37.6 1 5812.0 0.1 0.01 Av=5811.9kc [?]=0.023 Nuclevs Hg199(Sample Hg#4) (6775-6750kc) Time Dist Marker Res Dist Marker no. Res freg [?] [?] 31min 6306 15.4 1 6769.0kc 0 0 66 6209 15.2 1 6769.1 0.1 0.01 103 6308 16.0 1 6768.8 0.2 0.04 138 6301 14.6 1 6769.3 0.3 0.09 Av=6769.0kc [?]=0.046 vd2=5811.9+-0.15=5811.9(1+-206x10-5) vhg199=6769.0+-0.21=(1+-301x10-5) VHS199/VD2=1.1646794(1+-5.7x10-5)=1.164068(1+-6x10-5)
56
vHg199/vD2 = 1.16468 +- 0.00007 = 1.16468 +- 0.0001 uHg199 = (1.16468 +- 0.0001) (0.428235) ~ 0.499 uN Dropper 26.3 drops = 1 c.c.
58
ueff soln salts Co [illegible] 4.6-5.0 4.4-5.2 Mn [illegible] 5.3-5.96 5.85 Fe [illegible] 5.94 5.4-6.0 (Mn [illegible]/Co [illegible])2 = 1.28-1.42 ~ 1.35
59
Sept. 29 49 Mo-sample m.w. = 238.14 (A) 13.58 guess of K2M0o4 in 12 ml of H2O Mo #1 4.75 M of Mo 2 o M of Co(NO3)2 Mo #2 M of Mo 2 M of Co(NO3)2 (B) 17.46 gm of K2MoO4 m 18 ml of H20 Mo #3 407 M of Mo 2 M of Co (NO3)2 Mo #4 407 M of Mo 2 M of Co (NO3)2 Mo #5 407 M of Mo M of Co (NO3)2
62
Mn#1 0.18M KMnO4 Resonance at 6.87 MC 7.21 MC 0.8 M Co(NO3)2 Ho = 6600 grams Mn#2 0.151 gms KMn04 in 5c.c, soln 3c.c. of Co(No3)2(2 molar) in 5c.c. soln Molarity of Mn=0.151/5/0.158=0.191 Molarity of Co++=3/5x2=1.2 v(Na23)=8845.4+-0.2KC v(Mn55)=8290.0+-0.2KC Dec14, '49 checked by using GR No.271 v(Mn55)/v(Na23)=6.8411/7.3001=0.932124 v(Mn55)/v(Na23)=8290.0/8845.4(1+-3x10-5)=0.937210 M(Na23)/3=0.738875MN M(Mn55)=3.46241Mn(1+-0.01270) =+(3.4624+-0.0004)MN M(Na23)/M(H1)=0.26450+-0.01% M(H1)=2.79348(1+0.00018)
69
N#2 19 c.c. soln contains 12 grams of NH4NO3 2 10 c.c. of 3.85 M soln of MnCl2 Molarity of N = 16M Molarity of Mn [illegible] = 2M N#3 18 c.c. soln contains 12.5 grams NH4NO3 10 c.c. of 3.8512 M soln of MnCl2 Molarity of N = 17.4 M Mn [illegible] = 1 M N#4 12.5 grams NH4NO3 in 18 cc H20 ; 0.2 M MnNO3 N A 1.948 g Cr(NO3)3 9 H2O 1.948 x 42/400.2= 0.204 g B 1.832 g NH4NO3 1.832x28/80 = 0.64 g C 2.240 g NH3 2.240x14/17 = 1.84 g Ration of A to (B+C) = 0.204/2.48 = 0.082 Total nitrogen content in NH3 = 2.18 g. Total nitrogen content = 2.64 g. Ratio = 2.18/2.64 = 0.825 0.825/15 = 0.055